In the past, flavor development for e-liquids was a creative pursuit driven by consumer trends. Today, it’s a legal and scientific challenge. With the tightening of international e-cigarette regulations—particularly the European Union’s Tobacco Products Directive (TPD) and the U.S. FDA’s Premarket Tobacco Product Application (PMTA)—flavorings are under regulatory scrutiny like never before.
To achieve global market entry and consumer trust, flavor formulations must meet the dual challenge of delivering sensory appeal while remaining fully compliant with evolving legislation.
The TPD (Directive 2014/40/EU) doesn’t just govern nicotine levels or packaging—it regulates what you can and cannot put into the flavoring component of e-liquids:
Under the PMTA, manufacturers must provide scientific evidence that the flavoring used is “appropriate for the protection of public health.” This involves:
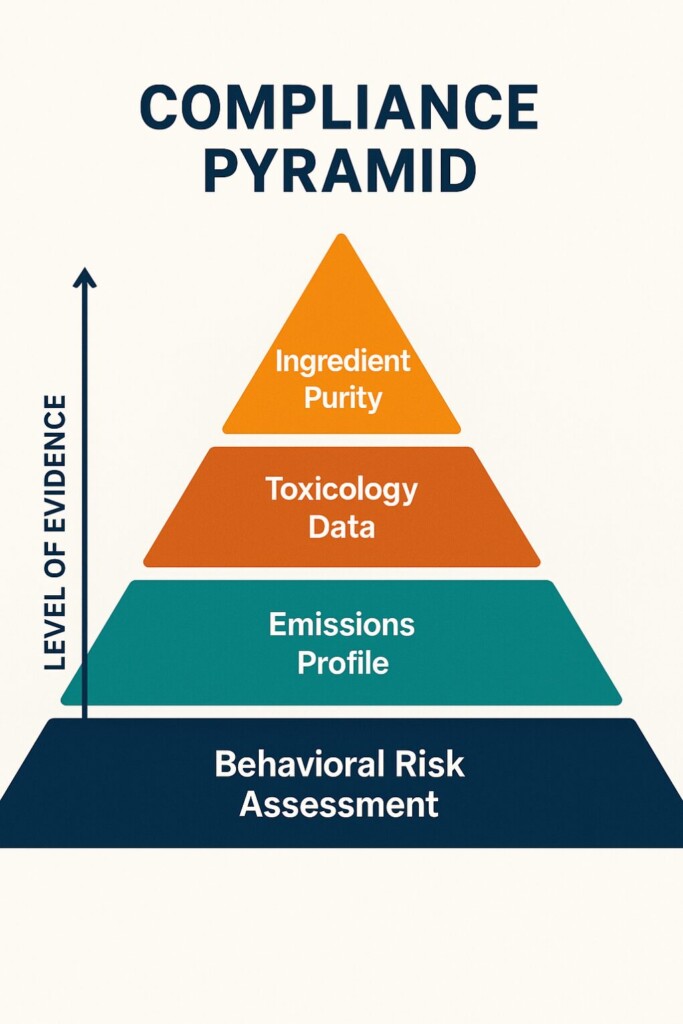
Even experienced formulators may unintentionally fail compliance tests. Let’s highlight some frequent mistakes:
Using unapproved carriers like Triacetin, Diacetyl, or Benzaldehyde in excess—even if flavor-enhancing—can trigger bans.
“Natural” doesn’t equal “safe.” Extracts must be:
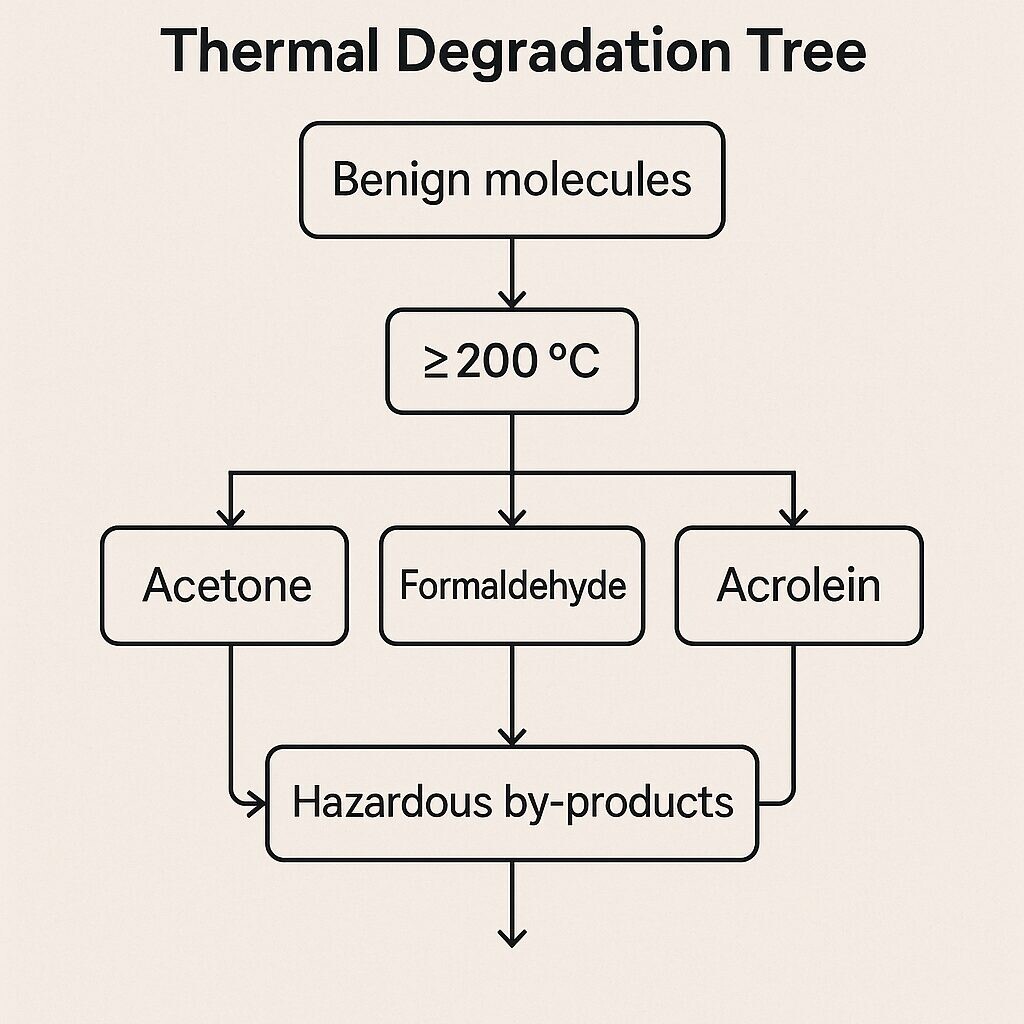
High-heat devices can degrade flavor molecules into toxic or unknown byproducts, such as:
While GRAS (Generally Recognized as Safe) status is a baseline, it is not enough. E-liquid flavorings should be:
Stay clear of:
Fewer components = lower risk. For PMTA in particular:
✅ Recommended Option: “CUIGUAI Flavoring offers pre-formulated, PMTA-ready flavorings that exclude restricted compounds while maintaining optimal sensory impact.”
Regulators demand evidence-based safety, not manufacturer claims. Make sure your flavoring meets the following:
Flavorings must withstand:
This iterative and evidence-driven workflow ensures flavors are not only delightful but legally durable.
Compliance will soon be proactive. With the rise of AI in flavor formulation and in silico toxicology, manufacturers can:
Advanced systems like digital twins of flavor matrices may revolutionize regulatory submissions by linking lab data to real-time compliance models.
As regulations become more comprehensive, designing e-liquid flavors is no longer a matter of creativity alone—it’s a complex balance of toxicological caution, regulatory precision, and sensory science.
CUIGUAI Flavoring supports manufacturers with a curated portfolio of regulatory-compliant, pre-tested flavorings designed for TPD, PMTA, and emerging standards. Build safer, smarter, and globally accepted flavors—without sacrificing taste.
Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.
Last Updated: May 29, 2025
The business scope includes licensed projects: food additive production. General projects: sales of food additives; manufacturing of daily chemical products; sales of daily chemical products; technical services, technology development, technical consultation, technology exchange, technology transfer, and technology promotion; biological feed research and development; industrial enzyme preparation research and development; cosmetics wholesale; domestic trading agency; sales of sanitary products and disposable medical supplies; retail of kitchenware, sanitary ware and daily sundries; sales of daily necessities; food sales (only sales of pre-packaged food).
Copyright ©Guangdong Unique Flavor Co., Ltd.All Rights Reserved. Privacy Policy